Answer:
A. 4+.
B. 4+
C. 2+.
Step-by-step explanation:
Hey there!
In this case, according to the given substances, it turns out possible for us to find the oxidation number of each element by applying the concept of charge balance in all of them as shown below:
A. sulfur in S032- : overall charge is 2- and the oxidation number of oxygen is 2-, thus:
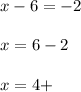
B. nickel in NiO2 : overall charge is 0 and the oxidation number of oxygen is 2-, thus:
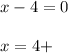
C. iron in Fe(OH)2: overall charge is 0 and the oxidation state of the OH ion is 1-, thus:
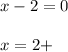
Regards!