Answer:
5.22*1022 molecules of glucose (C6H12O6) is equal to 15.08 grams
Step-by-step explanation:
Number of molecule of glucose in one mole
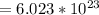
Now mass of one mole of glucose
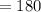 grams
grams
Number of moles in
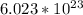 molecules of glucose
molecules of glucose
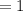
Number of moles in
 molecules of glucose =
molecules of glucose =
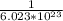
Number of moles in
 molecules of glucose =
molecules of glucose =
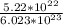 =
=
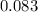 moles
moles
Weight of
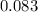 moles
moles
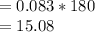 grams
grams