Answer:
The coefficient of carbon dioxide is eight (8).
Step-by-step explanation:
To balance the equation, we would have to ensure the number of chemical elements in the reactant side is equal to the number of chemical elements in the product side.
Given the chemical equation below;
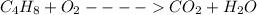
Now, we will balance the above equation with the smallest coefficients possible;
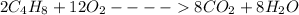
Let the Coefficient of Reactants be denoted by Cr and Coefficient of Product be Cp.
The Reactants are: C_{4}H_{8} and O_{2}
The products are: CO_{2} and H_{2}O
Therefore, we can deduce the following from the balanced chemical equation;
Cr(C_{4}H_{8}) = 2
Cr(O_{2}) = 12
Cp(CO_{2}) = 8
Cp(H_{2}O) = 8
Hence, the coefficients are 2, 12, 8 and 8 respectively.