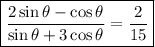9514 1404 393
Answer:
2/15
Explanation:
The given expression for θ can be rewritten to ...
5sin(θ + arctan(-3/4)) = 0
This will have solutions ...
θ +arctan(-3/4) = nπ
θ = nπ - arctan(-3/4) = nπ + arctan(3/4)
__
The corresponding values of sine and cosine will be ...
sin(arctan(3/4)) = 3/5
cos(arctan(3/4) = 4/5
or both values may be negative. Either sign will give the same result in the expression we're to find.
(2sin(θ) -cos(θ))/(sin(θ) +3cos(θ)) = (2(3/5) -(4/5))/(3/5 +3(4/5))
= ((6 -4)/5)/((3+12)/5) = 2/15