Answer:
V = 150 mL
Step-by-step explanation:
Hello!
In this case, since the molarity of a solution is computed via:
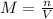
Whereas we are asked to compute the volume:
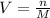
Thus, we first compute the moles in 12.0 g of NaOH as its molar mass is about 40.0 g/mol:
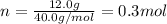
Thus, at first instance, the volume liters is:
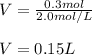
Which in milliliters is:
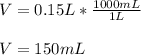
Best regards!