Answer:

Step-by-step explanation:
We want to find the mass percent composition of carbon in methane: CH₄
First, we must calculate the gram formula mass, also called the molar mass. Use the values for mass found on the Periodic Table. Look for carbon and hydrogen.
There is no subscript after C, so there is just 1 atom. There is a subscript of 4 after H, so there are 4 atoms of hydrogen. We must multiply hydrogen's mass by 4.
- C: 12.011 g
- H₄: 1.008 g * 4= 4.032 g
- CH₄= 12.011 g+ 4.032 g=16.043 g
Calculate the percent composition.
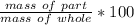
The part is the carbon, or 12.011 grams.
The whole is the entire compound, CH₄, or 16.043 grams.
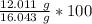
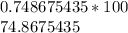
Let's round to the nearest hundredth. The 7 in the thousandth place tells us to round the 6 to a 7.
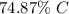
The mass percent of carbon is 74.87%