Answer:
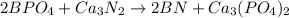
Double displacement.
Step-by-step explanation:
Hello!
In this case, according to the reactants, we need to keep in mind that boron has a charge of 3+, phosphate ion a charge of 3-, Calcium 2+ and nitride 3-. Thus, for the double displacement reaction that is analyzed, we obtain:
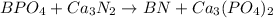
Which is balanced to:
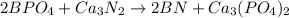
Best regards!