Given :
There are 0.090 moles of sugar (C₁₂H₂₂O₁₁) in the average can of soda.
To Find :
How many sugar molecules would this be ?
Solution :
We know, in 1 mole of any compound their are
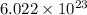 molecules of that compound.
molecules of that compound.
So, number of molecules in 0.090 moles of compound is :
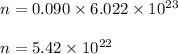
Therefore, number of molecules in 0.090 moles of compound is
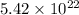 .
.