Answer:
1.03 grams of hydrogen is produced from 12.5 g of Mg reacting with hydrochloric acid.
Step-by-step explanation:
The balanced reaction is:
Mg+ 2 HCl → MgCl₂ + H₂
By stoichiometry of the reaction, the following amounts of moles of each compound participate in the reaction:
- Mg: 1 mole
- HCl: 2 moles
- MgCl₂: 1 mole
- H₂: 1 mole
Being the molar mass of each compound:
- Mg: 24.31 g/mole
- HCl: 36.45 g/mole
- MgCl₂: 95.21 g/mole
- H₂: 2 g/mole
By reaction stoichiometry, the following mass amounts of each compound participate in the reaction:
- Mg: 1 mole* 24.31 g/mole= 24.31 g
- HCl: 2 moles* 36.45 g/mole= 72.9 g
- MgCl₂: 1 mole* 95.21 g/mole= 95.21 g
- H₂: 1 mole* 2 g/mole= 2 g
Then you can apply the following rule of three: if by stoichiometry 24.31 grams of Mg produces 2 grams of H₂, 12.5 grams of Mg produces how much mass of H₂?
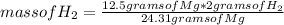
mass of H₂= 1.03 grams
1.03 grams of hydrogen is produced from 12.5 g of Mg reacting with hydrochloric acid.