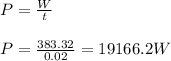Answer:
(a) 0.833 L
(b) - 383.32J
(c) - 383.32 J
(d) 19166.2 W
Step-by-step explanation:
initial volume, V = 3 L
initial temperature, T = 300 K
Initial pressure, P = 1 atm
final pressure, P' = 3.6 atm
Temperature is constant.
(a) Let the final volume is V'.
As the temperature is constant,
P V = P' V'
1 x 3 = 3.6 x V'
V' = 0.833 L
(b) Let the number of moles is n
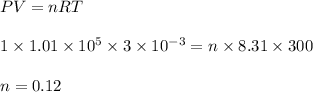
The work done in isothermal process is
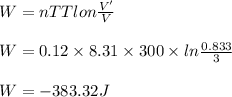
(c) The energy is given by the first law of thermodynamics
dQ = dU + dW
Here, dU is the zero as the temperature is constant.
So, the heat energy is
dQ = dW = - 383.32 J
(d) Time, t = 20 ms
Power is