Answer:
In a laboratory, a solution of sulfuric acid (H2SO4) was reacted with another of sodium hydroxide (NaOH), both aqueous. To form sodium sulfate (Na2SO4) and 2 molecules of liquid water (H2O). Complete in relation to the chemical equation that represents the reaction:
Step-by-step explanation:
The balanced chemical equation of the reaction is:
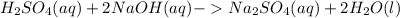
Thus, one mole of sulfuric acid reacts with two moles of sodium hydroxide.