Answer:
Option (d).
Step-by-step explanation:
According to the Boyle's law, for a given mass of a gas, the pressure of the gas is inversely proportional to the volume of the gas keeping the temperature of the gas is constant.
So,
Let the pressure is P, volume is V and T is the absolute temperature of the gas.
Pressure proportional to the reciprocal of the volume.
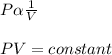
The correct option is (d).