Answer:
5.95g
Step-by-step explanation:
1
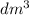 = 1000 mL
= 1000 mL
∴ 100 mL = 100 ÷ 1000 = 0.1
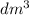
Volume = 0.1
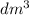
Concentration = 0.5 M
Concentration =
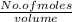
0.5 =
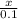
No. of moles = 0.5 x 0.1 = 0.05 moles
No. of moles =
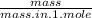
Mass in 1 mole of KBr = 39 + 80 = 119g (39 is the mass of potassium and 80 is the mass of bromine)
0.05 =
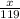
x = 119 × 0.05 = 5.95g