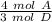Answer:
Step-by-step explanation:
The mole ratio in a balanced equation tells us the number of moles of each reactant needed and how many moles of product will be made.
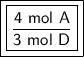
We are asked to find the mole ratio between A and D for this reaction.
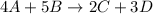
The mole ratio is found by comparing the coefficients, which are the numbers in front of the formulas.
A has a coefficient of 4 and D has a coefficient of 3.
Therefore, the ratio between A and D is:
- 4:3