Answer:
Hence among the options a and c, option d is that the correct answer because it has rock bottom energy ( as n value increases, energy decreases as energy levels come closer).
Step-by-step explanation:
The relation between energy and wavelength is:
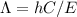
From this equation, it's clear that wavelength and energy are inversely proportional to every other. The Lower the energy of a specific transition, the longest will the wavelength be of that specific transition.
Among the given options, options b and d are often ruled out, since those transitions produce to release of a photon because it is coming down from an excited state.