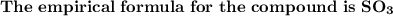Answer:
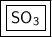
Step-by-step explanation:
An empirical formula shows the smallest whole-number ratio of the atoms in a compound.
So, we must calculate this ratio. Since we are given the amounts of the elements in moles, we can do this in just 2 steps.
1. Divide
The first step is division. We divide the amount of moles for both elements by the smallest amount of moles.
There are 0.300 moles of sulfur and 0.900 moles of oxygen. 0.300 is smaller, so we divide both amounts by 0.300
- Sulfur: 0.300/0.300= 1
- Oxygen: 0.900/0.300= 3
2. Write Empirical Formula
The next step is writing the formula. We use the numbers we just found as the subscripts. These numbers go after the element's symbol in the formula. Remember sulfur is S and there is 1 mole and oxygen is O and there are 3 moles.
This formula is technically correct, but we typically remove subscripts of 1 because no subscript implies 1 representative unit.