Answer:
the liquid has less height than the mercury
h_{ liquid} =
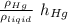
Step-by-step explanation:
The pressure as a function of the height is given by
P = ρ g h
where ρ is the density of the liquid, g the acceleration of gravity and h the height reached by the column of the liquid
In that case they say that the pressure is the standard one that is P = 1.01 10⁵ Pa = 760 mmHg
The first way to give the pressure is in SI units and the second way is the height that the mercury column reaches
In the case of building a barometer with a liquid that has a density greater than that of mercury
ρ_liquid > ρ_Hg
the pressure
P =ρ_lquid g h_liquid
if we have the same pressure
ρ_{Hg} g h_{Hg} = ρ_{liquid} g h_{liquid}
h_{ liquid} =
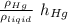
therefore the liquid has less height than the mercury