Answer:
30.8 grams of magnesium hydroxide will form from this reaction, and magnesium nitrate is the limiting reagent.
Step-by-step explanation:
The reaction that takes place is:
- 2NaOH + Mg(NO₃)₂ → 2NaNO₃ + Mg(OH)₂
Now we convert the given masses of reactants to moles, using their respective molar masses:
- 68.3 g NaOH ÷ 40 g/mol = 1.71 mol NaOH
- 78.3 g Mg(NO₃)₂ ÷ 148.3 g/mol = 0.528 mol Mg(NO₃)₂
0.528 moles of Mg(NO₃)₂ would react completely with (0.528 * 2) 1.056 moles of NaOH. There are more than enough NaOH moles, so NaOH is the reagent in excess and Mg(NO₃)₂ is the limiting reagent.
Now we calculate how many Mg(OH)₂ are produced, using the moles of the limiting reagent:
- 0.528 mol Mg(NO₃)₂ *
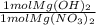 = 0.528 mol Mg(OH)₂
= 0.528 mol Mg(OH)₂
Finally we convert Mg(OH)₂ moles to grams:
- 0.528 mol Mg(OH)₂ * 58.32 g/mol = 30.8 g