Answer:
Option (B) is correct.
Step-by-step explanation:
Initial temperature of the gas is T.
The volume of the sample is decreased from 4.5 L to 1.5 L while the pressure is held constant.
At constant pressure, the relation between volume and temperature is given by :
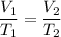
Here, V₁ = 4.5 L, V₂ = 1.5 L, T₁ = T₁, T₂ = ?
So,
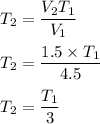
So, the final temperature of the gas is
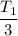 . Hence, the correct option is (B).
. Hence, the correct option is (B).