Answer:
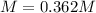
Step-by-step explanation:
Hello!
In this case, according to the following chemical reaction:
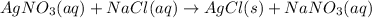
It is possible to compute the moles of silver nitrate via stoichiometry that produced 4.576 g of silver chloride as shown below:
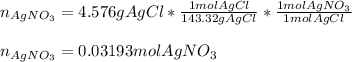
Thus, since the molarity is obtained by dividing moles by volume, we obtain:
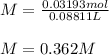
Best regards!