Answer:
11.84 moles
Step-by-step explanation:
We first balance the chemical equation
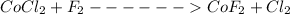
Both number of elements in the reactant side is equal to elements on the product side thus this equation is already balanced
Using mole ratio to find moles of
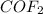
1 mole of CoCl2 reacts with 1 mole of F2 to produce 1 mole of CoF2 and ! mole of Cl2
Mole CoCl2 = Mole CoF2 = 11.84 moles