Answer: The statement conjugate base of hydrofluoric acid is weaker than that of acetic acid is most likely true.
Step-by-step explanation:
A strong acid upon dissociation gives a weak conjugate base. This can also be said as stronger is the acid, weaker will be its conjugate base or vice-versa.
Hydrofluoric acid is a strong base as it dissociates completely when dissolved in water.
For example,
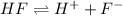
The conjugate base is
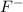 which is a weak base.
which is a weak base.
Acetic acid is a weak acid as it dissociates partially when dissolved in water. So, the conjugate base of acetic acid is a strong base.
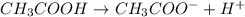
Thus, we can conclude that the statement conjugate base of hydrofluoric acid is weaker than that of acetic acid is most likely true.