Answer:
Step-by-step explanation:
Given that:
The flow rate Q = 0.3 m³/s
Volume (V) = 200 m³
Initial concentration
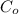 = 2.00 ms/l
= 2.00 ms/l
reaction rate K = 5.09 hr⁻¹
Recall that:
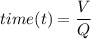
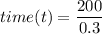
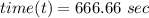
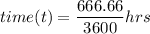
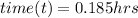
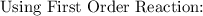
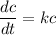
where;
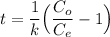
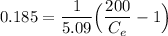
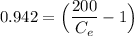
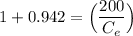
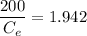
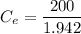
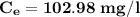
Thus; the concentration of species in the reactant = 102.98 mg/l
b). If the plug flow reactor has the same efficiency as CSTR, Then:
![t _(PFR) = (1)/(k) \Big [ In ( (C_o)/(C_e)) \Big ]](https://img.qammunity.org/2022/formulas/chemistry/college/gu782v5njl5l8on6b8jgbika47yz87k02p.png)
![(V_(PFR))/(Q_(PFR)) = (1)/(k) \Big [ In ( (C_o)/(C_e)) \Big ]](https://img.qammunity.org/2022/formulas/chemistry/college/vnjknzq5hth7rlqdsnlfyp16f9lpeqv46b.png)
![(V_(PFR))/(Q_(PFR)) = (1)/(5.09) \Big [ In ( (200)/(102.96)) \Big ]](https://img.qammunity.org/2022/formulas/chemistry/college/edlkcalw2ihl0ctosdt3veca9ubkyut9hk.png)
![(V_(PFR))/(Q_(PFR)) =0.196 \Big [ In ( 1.942) \Big ]](https://img.qammunity.org/2022/formulas/chemistry/college/b7jz2uf2xk70xwy7tpvsi6jaihbx3hg5ca.png)
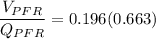
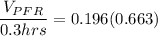
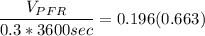
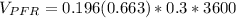
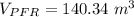
The volume of the PFR is ≅ 140 m³