Answer:
(6 mol)·(-393.5 kJ/mol) + (6 mol)·(-285.83 kJ/mol) - (1 mol)·(-1,273.02 kJ/mol) + (6 mol)·(0 kJ/mol)
Step-by-step explanation:
Question; From the given options, the chemical reaction in the question is presented as follows;
C₆H₁₂O₆(s) + 6O₂(g) → 6CO₂(g) + 6H₂O(l), given that we have;
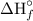 for C₆H₁₂O₆ = -1,273.02 kJ/mol
for C₆H₁₂O₆ = -1,273.02 kJ/mol
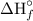 for O₂(g) = 0 kJ/mol
for O₂(g) = 0 kJ/mol
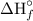 for CO₂(g) = -393.5 kJ/mol
for CO₂(g) = -393.5 kJ/mol
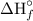 for H₂O(l) = -285.83 kJ/mol
for H₂O(l) = -285.83 kJ/mol
The heat or enthalpy of a reaction, is given as follows;
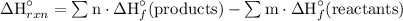
Therefore, the equation which should be used to calculate
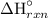 , is given as follows;
, is given as follows;
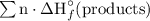 = (6 mol)·(-393.5 kJ/mol) + (6 mol)·(-285.83 kJ/mol)
= (6 mol)·(-393.5 kJ/mol) + (6 mol)·(-285.83 kJ/mol)
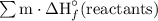 = (1 mol)·(-1,273.02 kJ/mol) + (6 mol)·(0 kJ/mol)
= (1 mol)·(-1,273.02 kJ/mol) + (6 mol)·(0 kJ/mol)
Therefore;
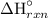 = (6 mol)·(-393.5 kJ/mol) + (6 mol)·(-285.83 kJ/mol) - (1 mol)·(-1,273.02 kJ/mol) + (6 mol)·(0 kJ/mol)
= (6 mol)·(-393.5 kJ/mol) + (6 mol)·(-285.83 kJ/mol) - (1 mol)·(-1,273.02 kJ/mol) + (6 mol)·(0 kJ/mol)