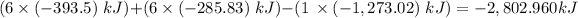Answer:
-2,802.960 kJ
Step-by-step explanation:
The dimensional equation of the given quantities is presented as follows;
(6 mol) × (-393.5 kJ/mol) + (6 mol) × (-285.83 kJ/mol) - (1 mol) × (-1,273.02 kJ/mol) = ? kJ
The equation, can be written as follows;
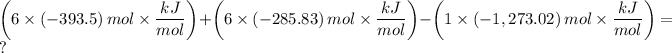 We note that
We note that
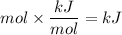 , therefore, the above equation becomes;
, therefore, the above equation becomes;