Answer:
The equilibrium constant of the mixture is not given hence I will provide a general answer
answer :
( new concentration at constant temp and volume of 6.79 L)
NH3 = 0.45 - X
HI = 0.28 - X
Step-by-step explanation:
Given data :
Equilibrium mixture volume = 16.2 L
temperature = 712 k
0.218 mol of NH4 I(s)
0.189 M NH3
0.116 M HI
Determine the concentrations of the two gases once equilibrium has been reestablished at a constant temperature and to a volume of 6.79 L
first step : calculate the number of moles for the gases
NH3 = 0.189 * 16.2 = 3.06 moles
HI = 0.116 * 16.2 = 1.88 moles
when the mixture is compressed to a volume of 6.79 L
the concentrations of the gases ( moles / volume )
NH3 = 3.06 / 6.79 = 0.45
HI = 1.88 / 6.79 = 0.28
Change in Concentrations of the two gases at compressed volume of 6.79 L at constant temperature can be expressed as
NH3 = 0.45 - X
HI = 0.28 - X
where X = K ( equilibrium constant ) =
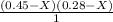 ----- ( 1 )
----- ( 1 )
Insert the value of K into equation 1 and solve for X
where : 1 = concentration of NH4 I(s)