Answer:
Molecular Formula: CCl4
Step-by-step explanation:
First, in order to find the molecular formula, we have to find the empirical formula.
We need to convert the percentages of each element to grams, and then moles. Usually, for problems like these, since we don't already have the chemical formula, we assume that the percents are in grams...
7.79% C becomes 7.79 grams of Carbon, and
92.21% Cl becomes 92.21 grams of Chlorine.
Now that we have grams, we can convert to moles.
The amu of Carbon is 12.01 g, and the amu of Chlorine is 35.45 g.
Carbon in moles =
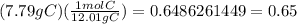
Chlorine in moles =
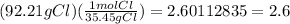
Now that we have the moles, we want to keep these as subscripts and write them into an empirical formula.
Since these numbers are in decimal formula, we have to divide them by the smallest mole in order to get a whole-number for our subscripts.
0.65/0.65 = 1, so the subscript for carbon is 1 (which can just be written as C with nothing next to it)
2.6/0.65 = 4, so the subscript for chlorine is 4 (which can be written as Cl4)
Now we will write our empirical formula as
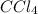 .
.
In order to find our molecular formula, we will take the molecular mass (aka the molecular formula's molar mass which is 152) and divide by the mass from the empirical formula. Then we will get another whole-number multiple that we will multiply our subscripts by in order the numbers for the subscripts for our molecular formula.
The total mass from the empirical formula is 153.81
[12.01 + 4(35.45) = 153.81]
152/153.81 = 0.9882322346 which can be rounded to 0.99 or just 1.0..
When we multiply our subscripts by 1.0, they equal 1 and 4 (the same numbers as before), which means that our empirical formula and molecular formulas are the same.
Empirical Formula: CCl4
Molecular Formula: CCl4