Answer: The mass in
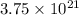 atoms of zinc is 0.405 g.
atoms of zinc is 0.405 g.
Step-by-step explanation:
Given: Atoms of zinc =
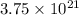
It is known that 1 mole of every substance contains
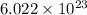 atoms. So, the number of moles in given number of atoms is as follows.
atoms. So, the number of moles in given number of atoms is as follows.
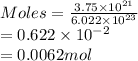
As moles is the mass of a substance divided by its molar mass. So, mass of zinc (molar mass = 65.39 g/mol) is calculated as follows.
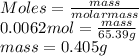
Thus, we can conclude that the mass in
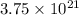 atoms of zinc is 0.405 g.
atoms of zinc is 0.405 g.