Answer:
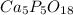
Step-by-step explanation:
Hello!
In this case, since the determination of empirical formulas requires the moles of the constituents, we first need to calculate the moles in the given grams of the listed elements:
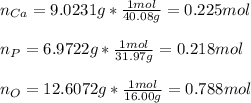
Next, we divide each moles by the fewest moles, in this case, those of phosphorous, in order to determine their subscripts in the empirical formula:
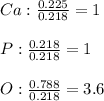
Thus, we multiply these subscripts by 5 to get whole numbers:
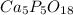
Best regards!