Answer:
The mass of bromine liquid that participates in a chemical reaction=12.8 g
Step-by-step explanation:
We are given that
Total number of moles of bromine liquid participate in chemical reaction=0.0800 moles
We have to find the mass of bromine liquid that participates.
Atomic mass of Br=79.9 g
1 mole of bromine liquid=2 atomic mass of bromine (Br)
1 mole of bromine liquid (
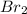 ) =
) =
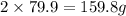
0.0800 moles of bromine liquid=
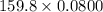 g
g
0.0800 moles of bromine liquid=12.784 g
0.0800 moles of bromine liquid
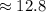 g
g
Hence, the mass of bromine liquid that participates in a chemical reaction=12.8 g