Answer:
A sample of salt has 1.74 moles of sodium chloride. how many formula units of the ionic compound are in the sample?
Step-by-step explanation:
Given, 1.74 moles of NaCl.
Since one mole of NaCl consists of ---
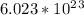 formula units.
formula units.
Then, 1.74mol of NaCl contains how many formula units of NaCl?
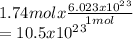 formula units.
formula units.
Hence, the given sample has 10.5x10^23 formula units.