Answer:
The combination of ions most likely to produce a precipitate is a group of answer choices:
lead nitrate soluble in water
Mg2+ and C2H3O2-.
Fe3+ and OH-.
Li+ and PO43-.
Pb2+ and NO3-.
NH4+ and SO42-.
Step-by-step explanation:
Among the given options,
magnesium acetate, lithium phosphate, lead nitrate, ammonium sulfate are soluble in water.
The only one which is insoluble in water is
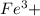 and
and
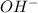 combination.
combination.
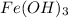 is insoluble in water. It forms a precipitate.
is insoluble in water. It forms a precipitate.