Solution :
For the reaction :
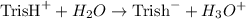
we have
![$Ka = \frac{[\text{Tris}^- * H_3O]}{\text{Tris}^+}$](https://img.qammunity.org/2022/formulas/chemistry/college/6cg0qskb88ztlp0yio93y5leqzrdv7rtgs.png)
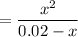
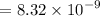
Clearing
 , we have
, we have
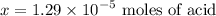
So to reach
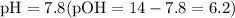 , one must have the
, one must have the
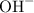 concentration of the :
concentration of the :
![$\text{[OH}^-]=10^(-pOH) = 6.31 * 10^(-7) \text{ moles of base}$](https://img.qammunity.org/2022/formulas/chemistry/college/n9h12op5vvtx8fw9q8a4qfusjuydgpvbcm.png)
So we can add enough of 1 M NaOH in order to neutralize the acid that is calculated above and also adding the calculated base.
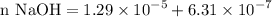
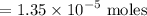
Volume NaOH
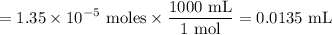
Tris mass
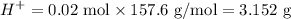
Now to prepare the said solution we must mix:
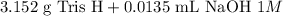 gauge to 1000 mL with water.
gauge to 1000 mL with water.