Answer:
Step-by-step explanation:
Given that:
At the inlet:
Temperature
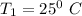
Pressure
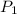 = 20bar = 2000 kPa
= 20bar = 2000 kPa
At the outlet:
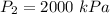
a)
Q = 500 kJ/kg
Using the steam tables to determine the properties of water;
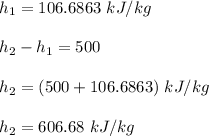
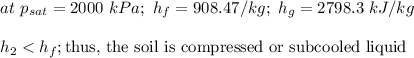
b)
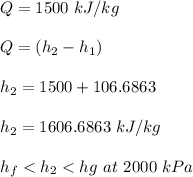
Thus, the outlet steam will be a vapor-liquid mixture at 212.38° C
c)
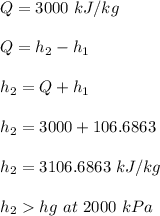
Thus, the outlet steam will be a superheated vapor at 380° C