Answer:
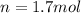
Step-by-step explanation:
Hello!
In this case, according to the ideal gas equation, it is possible to notice that:

Thus, since we are asked to compute moles, we proceed as follows:
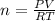
Now, since 1 L = 1 dm³ and 20 °C equal 293.15 K, we obtain:
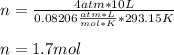
Best regards!