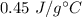Answer:
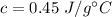
Step-by-step explanation:
Given that,
A 25 g sample of iron (initially at 800.00°C) is dropped into 200 g of water (initially at 30.00°C). The final temperature of the system is 40.22°C.
We need to find the specific heat of iron.
It can be calculated as:
Cooler water gains = hot metal loses
mc∆T = - mc∆T
Put all the values,
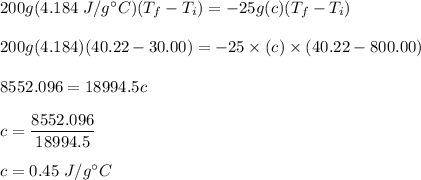
So, the specific heat of iron is