Answer:
Final temperature, T2 = 31.11°C
Step-by-step explanation:
Given the following data;
Mass = 4.5kg
Initial temperature = 20°C
Quantity of heat = 90000J
Specific heat capacity = 1,800. J/kg-°C
To find the final temperature;
Heat capacity is given by the formula;
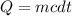
Where;
Q represents the heat capacity or quantity of heat.
m represents the mass of an object.
c represents the specific heat capacity of water.
dt represents the change in temperature.
Making dt the subject of formula, we have;
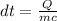
Substituting into the equation, we have;
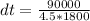
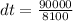
dt = 11.11°C
Now, to find the final temperature T2;
But, dt = T2 - T1
Substituting into the equation, we have;
T2 = 11.11 + 20
Final temperature, T2 = 31.11°C