Answer:
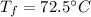
Step-by-step explanation:
Hello!
In this case, since this a problem in which the cold water is heated by the hot water, we can write:
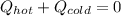
Thus, by plugging in the mass, specific heat and temperatures, we obtain:
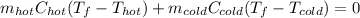
Now, we can also write:
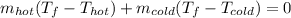
Then, after applying some algebra, it is possible to obtain:
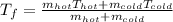
If we plug in, we obtain:
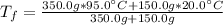
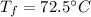
Best regards!