Answer: The given compounds are arranged according to decreasing boiling point as
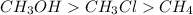 .
.
Step-by-step explanation:
The temperature at which vapor pressure of a substance becomes equal to the atmospheric pressure is called boiling point.
Stronger is the intermolecular forces present the atoms of a molecule more heat will be required by it to break the bond between its atoms. Hence, more will the boiling point of the molecule.
In
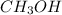 (methanol), there is hydrogen bonding present which is a stronger force. So, it will have highest boiling point as compared to
(methanol), there is hydrogen bonding present which is a stronger force. So, it will have highest boiling point as compared to
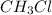 and
and
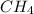 .
.
In
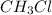 (chloroform), there is more electronegative atom attached (Cl) is attached to less electronegative atom (C and H). So, electrons are more pulled towards the chlorine atom. So, boiling point of
(chloroform), there is more electronegative atom attached (Cl) is attached to less electronegative atom (C and H). So, electrons are more pulled towards the chlorine atom. So, boiling point of
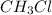 is more than methane
is more than methane
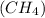 .
.
Thus, we can conclude that given compounds are arranged according to decreasing boiling point as
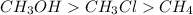 .
.