Answer: Among the listed substances
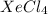 is the molecular compound.
is the molecular compound.
Step-by-step explanation:
A chemical compound formed by the chemical combination of two or more non-metals is called a molecular compound or covalent compound.
For example, Xe and Cl are non-metals. The compound formed by them is
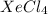 which is a molecular compound.
which is a molecular compound.
A molecular compound is formed by sharing of atoms between the combining atoms.
Whereas NaF,
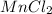 and CaO are all ionic compounds as they are formed by chemical combination of a metal and a non-metal.
and CaO are all ionic compounds as they are formed by chemical combination of a metal and a non-metal.
Thus, we can conclude that among the listed substances
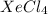 is the molecular compound.
is the molecular compound.