Answer: If matter goes through a chemical change then the physical properties are not likely to stay the same.
Step-by-step explanation:
When chemical composition of a substance changes during a chemical reaction then it is called a chemical change.
Chemical change always leads to the formation of new substances. Properties like chemical reactivity, combustion, rusting etc are chemical changes.
For example,
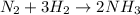
Here,
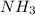 will have different chemical as well as physical properties as compared to
will have different chemical as well as physical properties as compared to
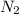 and
and
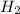 .
.
As physical properties are the properties that cause change in state of a substance.
Properties like boiling point, state of substance etc are physical properties.
Thus, we can conclude that if matter goes through a chemical change then the physical properties are not likely to stay the same.