Answer:
Current used = 1.90 A
Step-by-step explanation:
The Faraday's first law of electrolysis states:
M = ZIt
where: M is the mass of the element deposited, Z is the electrochemical equivalence of the element, I is the amount of current required and t is the time taken.
From the given question, M = 12.3 g, t = 5.5 h (19800 s) and Z = 0.0003296 g/C.
So that,
12.3 = 0.0003296 x I x 19800
12.3 = 6.52608 I
I =
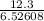
= 1.88475
I = 1.90 A
The value of the current used used is 1.90 A