Answer:
The temperature of the final equilibrium temperature of the system is 19.7⁰C
Step-by-step explanation:
Given;
mass of the ice, m₁ = 25 kg
temperature of the ice = 0°C
mass of the steam, m₂ = 4 kg
temperature of the steam, = 100 ⁰C
Let the temperature of the resulting mixture = t
Apply the principle of conservation of energy.
Heat required to melt the ice + Heat gained by the mixture = Heat required to convert the water to steam + Heat lost by the mixture
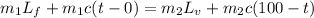
where;
Lf is the latent heat of fusion of ice = 3.33 x 10⁵ J/kg.
Lv is the latent heat of vaporization of water, = 2.260 x 10⁶ J/kg
c is the specific heat capacity of water = 4,200 J/kg
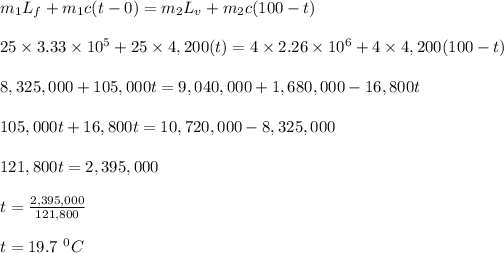
The temperature of the final equilibrium temperature of the system is 19.7⁰C