Answer:
the number of photons absorbed by the solar panel is 1.08 x 10²¹
Step-by-step explanation:
Given;
frequency of each photon absorbed, f = 5045 x 10¹⁴ Hz
energy to be created by the solar panel, E = 360 kJ = 360,000 J
The energy of each photon absorbed is calculated as;
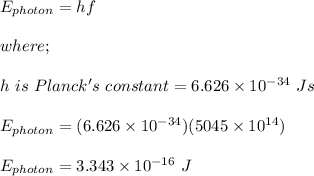
let the number of photons absorbed = n
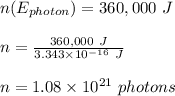
Therefore, the number of photons absorbed by the solar panel is 1.08 x 10²¹