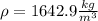Answer:
Step-by-step explanation:
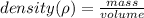
do note that you have to use the standardized units for both mass and volume.
mass - kilogram
volume -
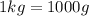
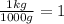
so if we have 18.4 g, then:
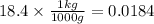
now for the volume

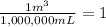
thus, 18.4 mL can be converted to m^3 through:

we can then find the density:
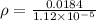
using a calculator: