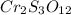Answer: The empirical formula of the compound becomes
Step-by-step explanation:
The empirical formula is the chemical formula of the simplest ratio of the number of atoms of each element present in a compound.
Let the mass of the compound be 100 g
Given values:
% of Cr = 26.53%
% of S = 24.52%
% of O = 48.96%
Mass of Cr = 26.53 g
Mass of S = 24.52 g
Mass of O = 48.96 g
The number of moles is defined as the ratio of the mass of a substance to its molar mass. The equation used is:
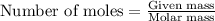 ......(1)
......(1)
To formulate the empirical formula, we need to follow some steps:
- Step 1: Converting the given masses into moles.
Molar mass of Cr = 52 g/mol
Molar mass of S = 32 g/mol
Molar mass of O = 16 g/mol
Putting values in equation 1, we get:
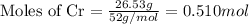
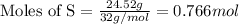
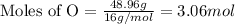
- Step 2: Calculating the mole ratio of the given elements.
Calculating the mole fraction of each element by dividing the calculated moles by the least calculated number of moles that is 0.510 moles
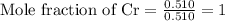
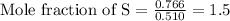
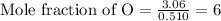
Multiplying the mole fraction of all the elements by 2, in order to make it as a whole number
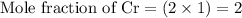
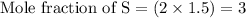
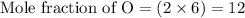
- Step 3: Taking the mole ratio as their subscripts.
The ratio of Cr : S : O = 2 : 3 : 12
Hence, the empirical formula of the compound becomes