By following these steps, you can systematically identify CO₂ in chemical reactions and make evidence-based claims about its presence.
To draw conclusions about the identity of carbon dioxide (CO₂) produced in chemical reactions, you should follow a systematic approach. Here's a step-by-step guide to help you identify CO₂ and provide evidence-based claims:
Step 1: Understanding the Basics of CO₂
- Chemical Formula:CO₂ consists of one carbon atom and two oxygen atoms.
- Properties: It is a colorless, odorless gas at room temperature, and is denser than air.
Step 2: Identifying the Reaction Types
CO₂ can be produced in various types of reactions. Common ones include:
- Combustion: Burning hydrocarbons (like methane, propane) in oxygen.
- Acid-Base Reactions: Reaction between carbonates or bicarbonates and acids.
- Respiration: In biological systems, CO₂ is a byproduct of cellular respiration.
Step 3: Examining the Reaction Equations
For each reaction, write down the balanced chemical equation. For instance:
- Combustion:
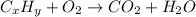
- Acid-Base:
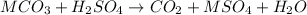 (where M is a metal)
(where M is a metal)
Step 4: Performing Experiments
Conduct experiments to see if CO₂ is produced. For instance:
- Limewater Test: Pass the gas through limewater (calcium hydroxide solution). If it turns milky, it indicates the presence of CO₂.
- pH Changes: CO₂ can acidify water, so testing the pH can be indicative.
Step 5: Observing the Results
- If the limewater turns milky, it is a strong indication of CO₂.
- Changes in pH toward acidity when the gas dissolves in water also suggest CO₂.
Step 6: Making an Evidence-Based Claim
Based on the experiments and observations, you can make a claim. For example:
- "In the combustion of methane, the formation of a milky precipitate in limewater and the observed decrease in pH when the gas dissolves in water strongly indicate the production of carbon dioxide."
Step 7: Discussing Possible Errors or Alternative Explanations
- Consider other gases that might react similarly and how you can differentiate them.
- Discuss experimental errors that could affect the results.