Answer:
0.469 L
Step-by-step explanation:
In order to find the minimum volume of 2.49 mol HCl(aq) required to dissolve 10.5 g of Al metal, we must first balance the chemical equation:
2Al(s) + 6HCl(aq) ⟶ 2AlCl3(aq) + 3H2(g)
We do this by making sure the amount of each element is equal on both sides of the equation.
We can then determine the amount of moles in 10.5 g of Al. We do this by dividing the mass by the molar mass:
10.5 g Al ÷ 26.98 g/mol = 0.389 mol Al
Now we can determine how many of moles of HCl(aq) will react:
0.389 mol Al ×
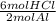 = 1.17 mol HCl
= 1.17 mol HCl
Now, we can finally find the volume of 2.49 mol/L HCl required for the reaction. We do this by dividing the amount of moles by the concentration:
1.17 mol HCl ÷ 2.49 mol/L = 0.469 L
You are correct!