Answer:
D) 1.61 times faster
Step-by-step explanation:
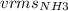 = √(3)RTM
= √(3)RTM
R constant= 0.08206
T=constant, so in this problem we dont need a value for it
M=17.031 g/mol
√(3)(0.08206)(17.031)= 2.047
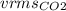 = √(3)RTM
= √(3)RTM
R constant= 0.08206
T=constant, so in this problem we dont need a value for it
M= 44.01 g/mol
√(3)(0.08206)(44.01)= 3.29
Since we are trying to measure how much faster NH3 will be, we have to realize that mass and speed have an inverse relationship.
So instead of doing (2.047)/(3.29) = 6.22
we have to flip the values to get (3.29)/(2.047)= 1.61