Molar mass is mass of 1 mole is approximately 25.98 grams
To determine the molar mass of the unknown gas, we'll follow these steps:
1. Convert Given Units to Appropriate Units for Ideal Gas Law
- Pressure: Convert from mmHg to atm (1 atm = 760 mmHg).
- Volume: Given in liters (L), which is appropriate.
- Temperature: Convert from Celsius to Kelvin (K = C + 273.15).
2. Use the Ideal Gas Law
The ideal gas law is given by PV = nRT , where:
- P is pressure in atm,
- V is volume in liters,
- n is the number of moles,
- R is the gas constant (0.0821 atm L/mol K),
- T is temperature in Kelvin.
3. Calculate the Number of Moles n
Rearrange the ideal gas law to solve for
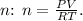
4. Calculate Molar Mass
Molar mass is the mass of 1 mole of the substance. It is given by:
![\[ \text{Molar Mass} = \frac{\text{Mass of the sample}}{n} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/c0bgt3un3z6hk3tq6sgupwk1xywy9jixf5.png)
Step-by-Step Calculations
Convert Units
- Pressure:
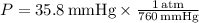
- Temperature:
T=25°C+273.15
Let's perform these conversions and then calculate the number of moles.
With the converted units, the pressure is approximately 0.0471 atm, and the temperature is 298.15 K.
5. Calculate the Molar Mass of the Gas
Now, using the calculated number of moles (0.0385 moles) and the given mass of the sample (1.00 g), the molar mass of the gas can be calculated as follows:
![\[ \text{Molar Mass} = \frac{\text{Mass of the sample}}{n} = \frac{1.00 \, \text{g}}{0.0385 \, \text{moles}} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/6y5iz5c4y52kszpjms7otchgsf7199sr0q.png)
Let's calculate the molar mass
The molar mass of the unknown gas is approximately 25.98 grams per mole.